Supporting your operations, from plant design expertise to equipment, parts and services for every stage of your process.
Are you looking to increase production, reduce risks, lower operating costs and enhance environmental performance? Then you are in the right place.
From the design and supply of products for a greenfield plant, to the addition of a single machine for an existing production line, we are here to help.
Rely on OEM experts because not all parts are created equal. Spare and wear parts built to perform.
Helping you get the most out of your equipment and processes.
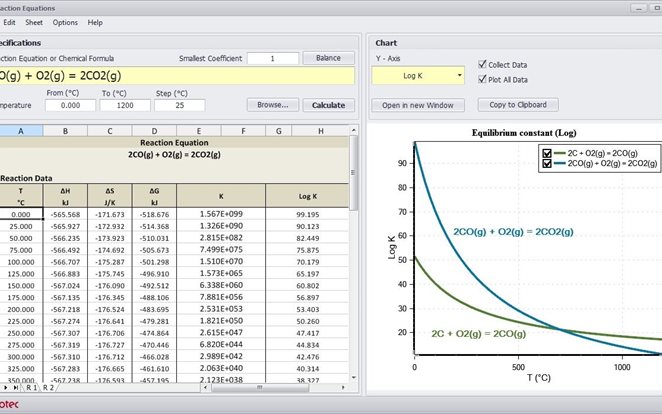
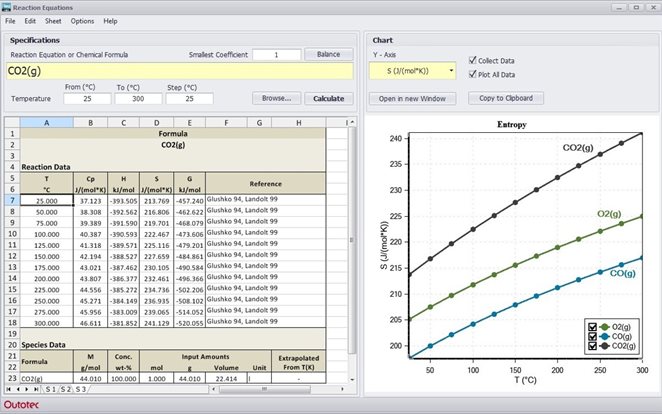
Reaction Equations Module does all the above instantly - you simply type the reaction equation in the input field and HSC gives you the heat of the reactions, the equilibrium constant at any temperature and the amount of species. HSC even checks the elemental balance and gives potentials vs. the standard hydrogen electrode for electrochemical reactions.
The calculated heat of reaction is the amount of heat that will be absorbed or released in the reaction. The equilibrium constant predicts the direction of the reaction. User input may be in the form of simple formulae or chemical reactions:
Na2SO4
Mg = Mg(g)
2Al + 3/2O2(g) = Al2O3
H2O = 1/2O2(g) + 2H(+a) + 2e-
Ag = Ag(+a) + e-
3NO2(-a) + H(+a) = 2NO(g) + H20 + NO3(-a)
2Al(+3a) + 3S(-2a) + 6H2O = 2Al(OH)3 + 3H2S(g)